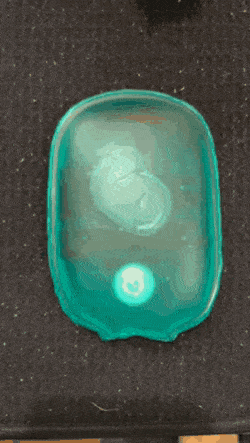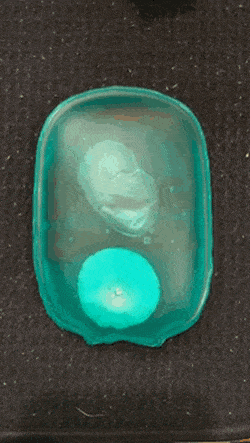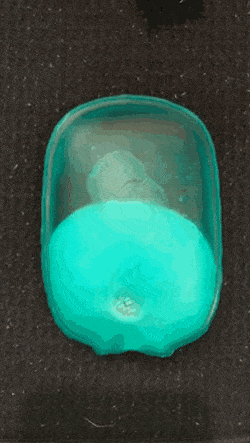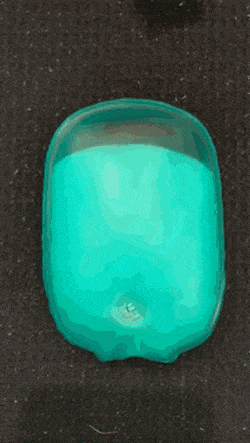
As we go into winter, it’s time to start pulling scarves and fluffy coats out the wardrobe, and keeping hand warmers in our pockets. If, like me, you like getting value for money, you might consider getting a reusable hand warmer instead of a one-time use one. They work quickly and effectively: simply snap the metal plate and wait for the crystals to grow and your hands to warm.
But how does it work? A wonder of chemistry, clearly. But some keywords are saturated (well, actually supersaturated), instability, crystalisation and exothermic. You can probably piece together a rough idea of what happens, so below I’m going to go into more depth and detail!

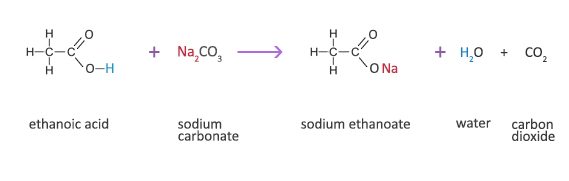
The clear solution inside the hand warmer is sodium acetate, CH3COONa, an organic sodium salt also called sodium ethanoate. It can be synthesised by reacting sodium with ethanoic acid. Hydrogen is bonded to an oxygen which is highly electronegative and draws electron density (from the bond) towards itself- this makes the hydrogen easily lost. This produces the acetate ion, CH3COO-, whose negative charge the sodium ion, Na+, is attracted to.

The sodium acetate (or sodium ethanoate, whichever you want to call it) is a supersaturated solution. As defined by trusty Wikipedia: “Supersaturation is a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances.”. To show by example, say you’re adding salt to water. You add a spoonful of your unbranded-for-legal-reasons salt and stir until so salt can be seen and then you add another spoonful and so on, until no more salt will dissolve no matter how much you stir. This is because the water has reached its solubility limit.

This can be increased by increasing the temperature of the water. If you tried dissolving salt in hot water and cold water, more salt will dissolve in the hot water. This is because in the hotter water, the particles have more kinetic energy. These particles (water molecules) can more easily break apart the solute molecules (the salt) by overcoming their intermolecular attraction.
So returning to hand warmers, sodium acetate is added to hot water so that more sodium acetate can be dissolved in it. This solution of sodium acetate and water is then cooled at a rate faster than the rate of precipitation, making the solution supersaturated.

Supersaturated solution are unstable and crystalise readily- these are properties that hand warmers utilise. When the metal plate (often made of stainless steel) in the solution is bent, one or more particle of metal are released. This starts off a reaction and for this reason, the metal plate can be called the activator. The metal particles which are released act as nucleation sites for crystals to form (otherwise called a nesting site).
The nucleation stage of crystallisation is the phase change in a small region, e.g. the formation of a solid crystal inside a liquid solution. There are two types of nucleation: homogeneous and heterogeneous. The kind in hand warmers is heterogeneous, which occurs when solid particles of a foreign substance increases the rate of nucleation (a.k.a trigger the fast crystallisation).
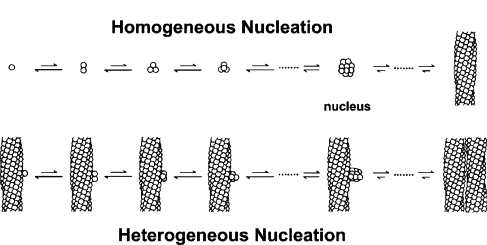
A crystallisation reaction occurs where many crystals are formed in a short period of time, releasing a large amount of heat in an exothermic reaction.
A hand warmer can be reset by leaving it in hot water. The hard warmer gains heat energy from the water and uses it to dissolve the salt in its own water of crystallisation. The (heat) energy required will be of a similar amount to the energy released when the metal plate is snapped.
(As a side note: Happy Halloween!)
Thanks for reading! You can check out some of my other posts here:
- Norimaki Synthesizer Taste Display: a tasty sample of science
- Applying to Cambridge Natural Sciences, a guide
- Michael Crichton, an author to remember
- Taking Lego down to near absolute zero with the first Lego cryonaut
- The standardisation of espresso flavour using maths
References
- https://en.wikipedia.org/wiki/Sodium_acetate
- https://en.wikipedia.org/wiki/Supersaturation
- https://en.wikipedia.org/wiki/Crystallization#Nucleation
- https://www.thoughtco.com/how-chemical-hand-warmers-work-607802
Hello! I’ve been following your weblog for a long time now and finally got the courage to go ahead and give you a shout out from Kingwood Texas! Just wanted to tell you keep up the excellent job!
LikeLike